45 fda guidance use of symbols on labels
FDA Issues Final Rule on Symbols for Device Labels | RAPS The US Food and Drug Administration (FDA) on Wednesday issued a final rule to allow for the use of standalone symbols on medical device and in vitro diagnostic (IVD) labels in an effort to align with international standards. In addition to allowing the use of standalone symbols, the final rule also permits the use of the symbol statements "Rx ... FDA Issues Final Rule Permitting Use of Symbols on Device Labeling So it is good news that FDA finalized the rule on June 15, 2016. Under the final rule, device manufacturers may use symbols on device labeling in one of the following ways: Continue using a symbol (s) with adjacent explanatory text. Use a symbol alone without adjacent explanatory text, so long as any one of the following conditions are met:
Use of Symbols in Labeling: Frequently Asked Questions | FDA Yes. The final rule explicitly allows for the optional use of stand-alone symbols in medical device labeling under certain circumstances. We do not expect manufacturers to change existing labeling ...
Fda guidance use of symbols on labels
FDA ANNOUNCES GUIDANCE FOR SYMBOL USE ON IVD LABELS - FDAnews The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices. EOF Draft Guidance for Industry and FDA Staff; Use of Symbols on Labels and ... EN 980, Graphical Symbols for Use in the Labeling of Medical Devices. This document provides guidance on the use of those recognized symbols. II. Significance of Guidance. This draft guidance is being issued consistent with FDA's good guidance practices regulation (21 CFR 10.115). The draft guidance represents the agency's current thinking on ...
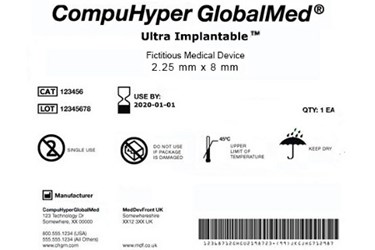
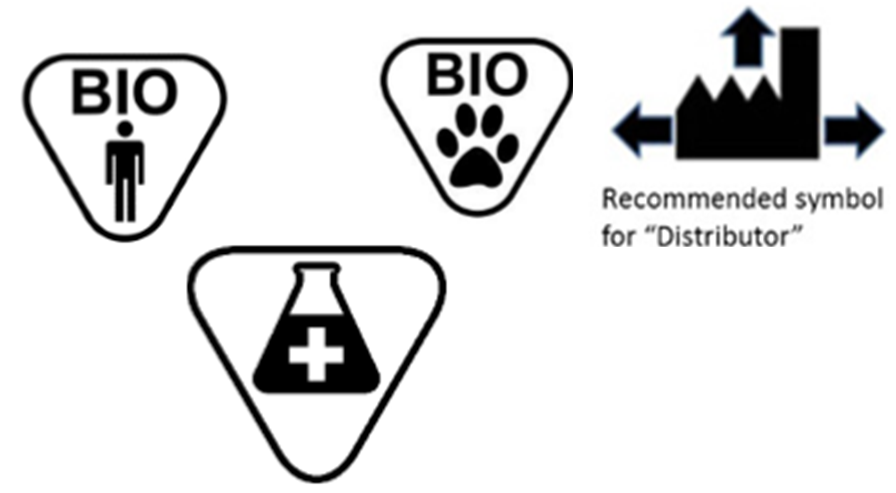
Fda guidance use of symbols on labels. Federal Register :: Use of Symbols in Labeling If an FDA-allowed stand-alone symbol is used, for example, in place of the wording "manufacturer:" or "manufacturing site:" followed by a name and address, the final rule requires that a symbols glossary must be included in the labeling for the device to explain the meaning of the symbol to the device's user. Fda Announces Guidance for Symbol Use on Ivd Labels The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices. FDA Final Rule on the Use of Symbols in Labeling - loring 1. The symbol is established in a standard [that] is recognized by the FDA, or. 2. The device manufacturer determines that the symbol is likely to be read and understood by the ordinary individual under customary conditions of purchase and use. A symbols glossary is not required for symbols used in labeling with adjacent explanatory text. Use of Symbols in Labeling | FDA The Food and Drug Administration (FDA) issued a final rule, Use of Symbols in Labeling, June 15, 2016, that became effective September 13, 2016. The final rule permits the use of symbols in all ...
PDF Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Device ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553. Draft Guidance for Industry and FDA Staff; Use of Symbols on Labels and ... EN 980, Graphical Symbols for Use in the Labeling of Medical Devices. This document provides guidance on the use of those recognized symbols. II. Significance of Guidance. This draft guidance is being issued consistent with FDA's good guidance practices regulation (21 CFR 10.115). The draft guidance represents the agency's current thinking on ... EOF FDA ANNOUNCES GUIDANCE FOR SYMBOL USE ON IVD LABELS - FDAnews The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices.

![PDF] Signs of change or clash of symbols? FDA regulation of ...](https://d3i71xaburhd42.cloudfront.net/103393b061f49e3976a4eaa8b35a160bfa9b2f8a/12-Figure1-1.png)













.png.aspx)












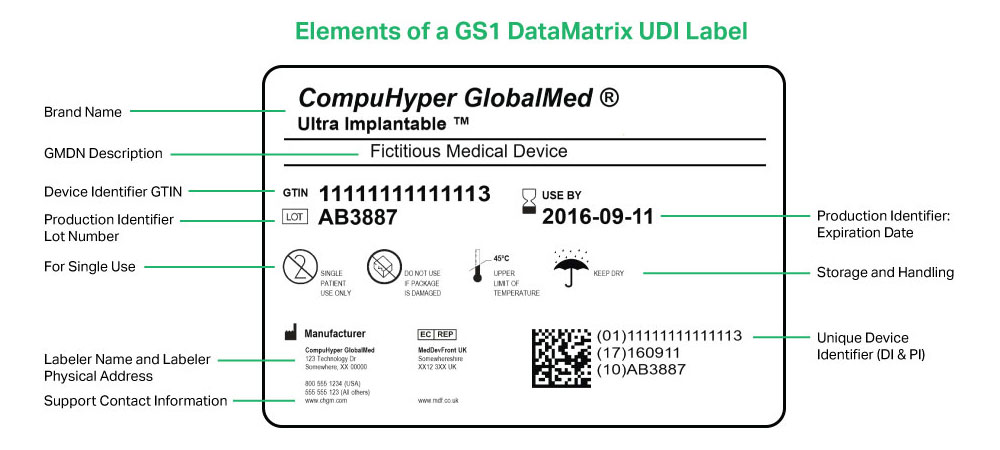

Post a Comment for "45 fda guidance use of symbols on labels"